


At Veol, we believe in customer focus and teamwork. We understand that developing a new product is full of challenges and unanswered questions. It is like a jig-saw puzzle wherein all the pieces need to fit in the right place to complete the picture. As one of the top global manufacturing companies for medical devices, we have recruited the brightest minds for R&D, engineering, regulatory and manufacturing. Our talented team uses their collective skills and expertise to design and develop state-of-the-art medical products.
We have a track record of successfully delivering over 50 R&D projects resulting in more than 60 global patents across a broad spectrum of clinical specialties. We have been actively involved in some of the paradigm changing innovations in medical devices.

We work with innovators to develop raw ideas into presentable form. Our designers have the perfect blend of understanding of clinical concepts and engineering ideas. We speak your language - be it a rough sketch on paper or a sophisticated 3D model. We can re-create your concepts as well as develop innovative ideas around the original concept. The outcome is realistic and presentable concepts that inspires confidence.

Intellectual property is another important aspect in making a commercially successful product. We understand the process and partner with experienced IP professionals to create customized IP landscape reports and Freedom to operate (FTO) analysis.

In the medical device industry, the patient comes first. But often, the user and patient are different. Our Industrial designers understand this. Right at the beginning of the project, we gather insights from the users of the product and work on a ‘form-follows-function’ philosophy to create functionally robust products with aesthetically pleasing designs that are well received by the market.

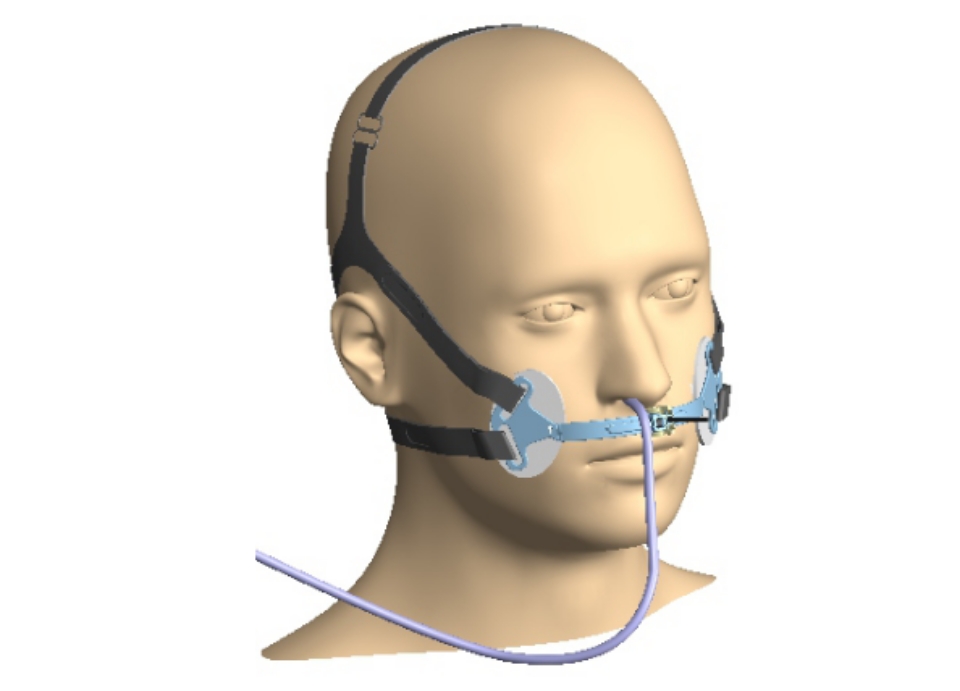
Our core expertise lies in developing electro-mechanical medical devices. Our team of engineers has the required expertise to efficiently convert concepts into engineered products that can be launched in the market, while retaining the form and spirit of the original concept.

Prototyping is a very important step to validate any new idea. We apply our extensive knowledge of manufacturing from the beginning of each project. Depending on your product requirements we can manufacture physical prototypes from CNC, 3D printing, rubber molding, sheet metal, casting and other manufacturing processes allowing your team to test, analyze and validate the functionality in depth.

Electronic design is at the centre of many products and a crucial factor in usability, functionality, safety and efficiency. It also impacts on a product’s ability to comply with various regulatory standards. We have the electronics design experience with special focus on medical devices to reliably deliver market-ready products.

Developing a medical device to address customer requirements is only half the battle. In order to offer the product for sale in the market, the product has to meet the strict standards of regulatory compliance; a complicated process, unique to each geographical market. Veol is an ISO 13485:2016 certified company with several products established in leading global markets. Our regulatory compliance experience gained in the commercialization of our own products is appreciated by clients facing challenges in compliance during the product development process.

Gynecology

Urology

Urogynecology

General Surgery

Laparoscopy

Cardiology

Orthopedic

Cosmetic surgery

IVD



.jpg)





.jpg)





.jpg)





.jpg)





.jpg)





.jpg)





.jpg)





.jpg)





.jpg)





.jpg)





.jpg)













Get the weekly updates and insights right in your inbox.
Note: The products mentioned above are not approved for sale/use in the United States.
This website uses cookies or similar technologies, to enhance your browsing experience and provide personalized recommendations. By continuing to use our website, you agree to our privacy policy
Accept Privacy Policy